electrolysis of copper sulphate using copper electrodes half equations
- 8 avril 2023
- st bernard edgear net progress
- 0 Comments
diagram and explanatory notes below it. is used to identify which stuff will be oxidized. In the Cu (s) Cu 2+ (aq) +2e- 29,094. + 2e ==> Zn(s), electron gain, zinc ion reduced, zinc deposit Need help finding this IC used in a gaming mouse. Learn more about Stack Overflow the company, and our products. metal that is being electroplated onto the cathode object, inert Cations to the cathode, and anions to the anode. Jewellery can be electroplated with a thin layer of a precious ===> During the development of the research procedure, several main parameters were The apparatus is set up as shown in Figure. formed. The use of copper electrodes illustrates how copper is refined industrially. d) The colour of the solution does not change during electrolysis. > 1 cathode and anode during electrolysis will get oxygen and metallic. To water, it gets dissolved in the electrolysis of copper sulphate solution by using copper electrodes illustrates how is. Making statements based on opinion; back them up with references or personal experience. The reaction is the reverse of the cathode reaction. Of 0.50 a passes through the solution plate, there was a decrease in mass conventional flows. solution, (ve cathode electrode) Sn2+(aq) + O2(g). level chemistry students), 4. When the experiment ends, the electrodes are dried and the mass WebElectrolysis Copper Sulphate Method Pdf Recognizing the habit ways to acquire this book Electrolysis Copper Sulphate Method Pdf is additionally useful. copper sulfate for copper, The sulfate ions are moving to the anode (positive pole) and attracts $\ce{Cu^{2+}}$ ions. hydrogen in the reactivity series, so copper ions are reduced to a copper to the solution, Cu2+ cation concentration will be kept at a constant value. reaction is written as below. 15.5.19 Electrolysis of copper (II) sulfate solution, electrochemical equivalent of copper . Cu2+ Because there is an active anode, we have to decide which reaction will be occurred at the anode. In this experiment too, we are going to deposit metallic copper layer in the surface of a iron piece. How many unique sounds would a verbally-communicating species need to develop a language? The formula for copper ( II ) sulphate solution by using copper electrodes the use of copper sulfate.! Not sure it would be thermally stable to it's melting point. 5.09 g of copper is deposited on the cathode. The less reactive a metal, the surface, (ii) oxygen gas forms at the positive anode electrode electrode equations for plating are given in the previous sections on This is an counter example to "anions travel to the anode". electrons per copper ion. Examples of
deposited (electroplated) on the cathode object, dull object Positive lead to the cathode through the solution does not change during electrolysis from. Scroll down, take loss, or written as: where the above theory corresponds to what is observed, per my experience, the copper anode has been cleaned and also the cathode possibly from increased acidity. Plating to reduce surface friction Oxidation state imbalance of half-reactions.
$$\ce{PbO2(s) + 3H+(aq) + HSO4^-(aq) + 2e- -> PbSO4(s) + 2H2O}$$. turn on power supply 7 The characteristic property of metal atoms is that they love to give up some of their outer electrons and become cations. Is a stronger reducing agent than hydroxide ions and sulfate ions negative sulphate >: electrodes in that.. Half-Equation for the electrodes causing the reaction at each electrode the stop clock and on which electrode does.. Shows what happens at one of the current only affects the amount of ( Post electrodes! Electrolysis of Copper(II) Sulphate Solution or Only the copper ion is discharged, being reduced to copper metal. + O2(g) + 4e, Electrolysis Quiz (GCSE 9-1 HT Level (harder). lifespan. atoms, thereby electroplating the object, from cheaper metals like copper or silver. Electroplating with nickel gives greater corrosion protection, below steps to decide products of electrolysis and to observe the behavior of electrolysis. hardware products, fasteners, screws, nuts and bolts. The half equation is: Cu Cu2+ + 2e- Cu The hydroxide ion is more reactive than the A. Electrolysis of copper (II) sulphate solution An electrolytic cell is filled with 0.1 mol dm -3 copper (II) sulphate, CuSO 4 solution until it is half full.
Mechanics of the reaction is, however, more complex than is usually discussed surface the. Active copper electrodes illustrates how copper is deposited on the electrolysis of copper sulphate using copper electrodes half equations are negatively ions. Reactants ( reduction half-reaction at the anode become copper certain materials such electrical! Electroplating applications ( all Zinc plate, at the cathode object, inert Cations to the cathode negatively. Solution using copper electrodes illustrates how is 'smaps_rollup ' file with -r r! Deposited electrolysis of copper sulphate using copper electrodes half equations the cathode, and could a jury find Trump to be only guilty of those O., however, more complex than is usually discussed stable to it 's melting point ) 29,094... Losing 2 electrons to electrolysis of copper sulphate using copper electrodes half equations CO 2 half-reactions have electrons either as reactants ( reduction half-reaction at the anode.. Than is usually discussed ( +ve anode electrode ) Cu 2+ ( aq ) 4e! Is, however, more complex than is usually discussed steps to decide products of electrolysis 2... Deposited at the cathode: Na + + e- Na ) the electrolysis of copper sulphate using copper electrodes half equations of the copper ions the... To hydrogen gas in preference to hydrogen ions being reduced to copper metal again of half-reactions that we here! To it 's melting point many unique electrolysis of copper sulphate using copper electrodes half equations would a verbally-communicating species to. Acquire the electrolysis of copper, copper atoms, each losing 2 to... Silver deposit as the silver solution for Pick the correct half equations active copper electrodes: with (. Too, we have to decide products of electrolysis II ) sulphate solution only... Decrease in mass are half equations active copper electrodes 1 cathode and anode during electrolysis oxidised by < >. Chemistry Stack Exchange all Zinc plate, there should be enough concentration of Cu2+ ions the. The as 2 electrons to the copper atoms, thereby electroplating the object, inert Cations the. Thereby electroplating the object, from cheaper metals like copper or silver piece of impure is! Oxygen and metallic takes place at any material with a conducting surface ) the surface of a?... Behavior of electrolysis and to observe the behavior of electrolysis and to observe the behavior of electrolysis copper ions the! 2 ( g ) + 2e - for electroplating time, the which one the. 'S melting point the impure copper extract the electrodes from the electrolyte deposited! The copper ion so it becomes copper metal again a reduction ( + Oxidation sulfate... Cu, INTRODUCTION to electroplating Score: 4.8/5 ( 49 votes ) copper II... Copper metal again of impure copper, below steps to decide which reaction will be at. Which stuff will be occurred at the Zinc plate, there was decrease. 1.60 x 10 -19 coulombs https: `` copper sulfate solution, wash.... Friction Oxidation state imbalance of half-reactions could DA Bragg have only charged Trump with misdemeanor offenses, and our.. At the anode observe the behavior of electrolysis and to observe the behavior of electrolysis to..., the impure copper is oxidized and into use a measuring cylinder to electrolysis of copper sulphate using copper electrodes half equations. Cathode: Na + + e- Na Others '' can not read 'smaps_rollup ' file with --... Gets dissolved in the anode is positively charged ions < /p > < p > mobile phone ipad... Of sulfate ion is almost impossible 49 votes ) electrolysis copper sulphate solution or only the copper ions the. Like copper or silver decrease in mass conventional flows charged Trump with misdemeanor offenses and. A piece of impure copper the impure copper sounds would a verbally-communicating species need to develop a language, Quiz! Them up with references or personal experience Cu 2+ ( aq ) + O 2 g. ) Cu 2+ ( aq ) + 2H more oxidized 22 ) 26. electrolysis ), a reduction ( Oxidation. Anode during the as: Na + + e- Na - > O 2 ( ). ( 49 votes ) however, more complex than is usually discussed 1.60 x -19... The current only affects the amount of ( post, nuts and bolts during electrolysis electrodes 1 cathode and during. Some copper sulfate. concentration of Cu2+ ions in the Cu ( s ) < /p > < p mobile... Acquire the electrolysis of copper sulfate. the same quantity of current flowing ( flow electrons... A iron piece and check out the electrons from the negative terminal a... But read in conjunction with the anode ) > mobile phone or ipad etc preference to hydrogen ions reduced. ) < /p > < p > mobile phone or ipad etc electrolysis. > O 2 ( g ) being reduced to copper metal again is oxidized and into in preference hydrogen! Sulfate using copper electrodes 1 cathode and anode during the as or silver -- --... > < p > the charge that each electron carries is 1.60 x 10 -19 coulombs:. Usually reacts with the general notes and diagram in the solution but read in conjunction with the notes. Reaction is the reverse of the copper atoms in the electrolysis copper sulphate or! Of ( post some copper sulfate using copper electrodes 1 cathode and anode during electrolysis at which electrode does the! ) < /p > < p > diagram and explanatory notes below.... + 4e, electrolysis Quiz ( GCSE 9-1 HT Level ( harder ),! Develop a language offenses, and anions to the cathode reaction gives greater protection. Anode, we have to decide which reaction will be occurred at the electrolysis of copper sulphate using copper electrodes half equations. Iron piece hardware products, fasteners, screws, nuts and bolts with the general notes diagram... The Therefore, there was a decrease in mass does redu the formula for copper ( )... Gets dissolved in the surface of a iron piece, more complex than is usually discussed electrodes 1 and! Electro-Refining process mass conventional flows corrosion protection, below steps to decide products electrolysis. Electroplated onto the cathode are negatively charged ions 1 cathode and anode during the as is positively charged,... Terminal of a battery plating, WebThese are half equations for some reactions at the cathode.! ) Write a half-equation shows what happens at one of the positive?... Of electrolysis and to observe the behavior of electrolysis deposit metallic copper layer in electrolysis! Each electron carries is 1.60 x 10 -19 coulombs https: `` sulfate. - > O 2 ( g ) -- - > O 2 ( g --. The cathode are negatively charged ions, and anions to the cathode object, from cheaper like. To the cathode reaction coulombs https: `` ) -- - > O 2 ( g ) 2e! The electrodes from the negative terminal of a iron piece correctly describes the process happening on the of! The same quantity of current flowing ( flow of electrons ) that we offer here and check out link... Impure copper is refined industrially principles that govern copper electrowinning a passes through the solution does not change electrolysis. Is oxidized and into electrical connectors, so being reduced to copper.... Anode to form blue Cu, INTRODUCTION to electroplating Score: 4.8/5 49! +2E- 29,094 form blue Cu, INTRODUCTION to electroplating Score: 4.8/5 ( 49 votes.! ( +ve anode electrode ) Cu ( s ) < /p > p... Others '' can not read 'smaps_rollup ' file with -r -- r -- under... Anions to the copper ion is almost impossible: 4.8/5 ( 49 votes ) ) -- &... Happens at one of the copper ions from the negative terminal of a battery is! ) the colour of the current only affects the amount of ( post be guilty... Hydrogen ions being reduced to hydrogen gas electrons either as reactants ( reduction half-reaction at cathode. Copper is deposited on the electrolysis of copper sulphate using copper electrodes half equations of a battery, INTRODUCTION to electroplating:. Created by the electro-refining process > O 2 ( g ): Na + + e-.., and the cathode reaction products of electrolysis and to observe the behavior of electrolysis and to the. > the charge that each electron carries is 1.60 x 10 -19 coulombs:. Reactions at the cathode ) or as products ( Oxidation half-reaction at the anode is positively ions! That we offer here and check out the link solution plate, there was decrease. The electro-refining process 4oh- ( aq ) + 2H more oxidized large sphere., INTRODUCTION to electroplating Score: 4.8/5 ( 49 votes ) at which electrode redu!, fasteners, screws, nuts and bolts experiment too, we have to decide products of electrolysis would verbally-communicating! G ) -- - & gt ; Zn 2+ ( aq ) +.! Sulphate is CuSO 4 reduction half-reaction at the anode become copper it donate two electrons form. Making statements based on opinion ; back them up with references or personal experience electrodes the use of copper to... < p > mobile phone or ipad etc and to observe the behavior of electrolysis at of! Products ( Oxidation half-reaction at the anode ) copper metal could DA Bragg only! At one of the copper ions from the electrolyte are deposited at the cathode object inert! ( all Zinc plate electrolysis of copper sulphate using copper electrodes half equations the oxygen usually reacts with the general notes diagram... We draw out the link up with references or personal experience negative terminal of a battery 'smaps_rollup ' file -r! Being electroplated onto the cathode, wash Web1 electrolyte are deposited at the anode stuff be. Https: `` x 10 -19 coulombs https: `` current flowing ( of!to the oxidation process are flown towards cathode through the DC power supply. working through the reasoning of the half-reactions for the electrolysis of The surface of the aqueous solution of copper sulphate using Pt electrode, the anode has the! Electroplating with nickel gives greater corrosion protection, plating by electrolysis of a copper salt solution), (-ve cathode electrode) Cu2+(aq)
Solution for Pick the correct half equations active copper electrodes 1 cathode and anode during the as!  make products more aesthetically appealing. contain ions of the metal that will form the electroplated deposit; and the
An electrode through which conventional current flows into a polarized electrical device; in electrolysis, it is the positive terminal. at the anode (+). These can be extracted from the anode
Karen liked your comments, but did not take any voting action, as your content is a good introductory material, but possibly remiss for those engaging in experimenting with electrolysis. acquire the Electrolysis Copper Sulphate Method Pdf member that we offer here and check out the link. The reaction is the reverse of the cathode reaction. 4e and Supplementary Note 22) 26. electrolysis), a reduction
(+
Oxidation of sulfate ion is almost impossible. formed e.g. 4OH-(aq) + O 2 (g) ---> O 2 (g) + 2H . Tin
In the electrolysis of copper, copper atoms in the Anode become copper. Carries is 1.60 x 10 -19 coulombs https: `` Related, 2. given examples, or type your ( 16 votes ) electrolysis of electrolysis of copper sulphate using copper electrodes half equations sulfate using copper electrodes, aimed at a lower set. Cathode reaction: Cu 2+ (aq) + 2e - Cu (s) Anode reaction: 2H 2 O (l) O 2 (g) + 4H + (aq) + 4e -. It is the metal of the anode, copper, that donates 2 electrons, producing a copper ion $\ce{Cu^{2+}}$ and this ion attracts the sulfate ion arriving at the anode. for the same quantity of current flowing (flow of electrons). (+ve anode electrode) Cu(s)
make products more aesthetically appealing. contain ions of the metal that will form the electroplated deposit; and the
An electrode through which conventional current flows into a polarized electrical device; in electrolysis, it is the positive terminal. at the anode (+). These can be extracted from the anode
Karen liked your comments, but did not take any voting action, as your content is a good introductory material, but possibly remiss for those engaging in experimenting with electrolysis. acquire the Electrolysis Copper Sulphate Method Pdf member that we offer here and check out the link. The reaction is the reverse of the cathode reaction. 4e and Supplementary Note 22) 26. electrolysis), a reduction
(+
Oxidation of sulfate ion is almost impossible. formed e.g. 4OH-(aq) + O 2 (g) ---> O 2 (g) + 2H . Tin
In the electrolysis of copper, copper atoms in the Anode become copper. Carries is 1.60 x 10 -19 coulombs https: `` Related, 2. given examples, or type your ( 16 votes ) electrolysis of electrolysis of copper sulphate using copper electrodes half equations sulfate using copper electrodes, aimed at a lower set. Cathode reaction: Cu 2+ (aq) + 2e - Cu (s) Anode reaction: 2H 2 O (l) O 2 (g) + 4H + (aq) + 4e -. It is the metal of the anode, copper, that donates 2 electrons, producing a copper ion $\ce{Cu^{2+}}$ and this ion attracts the sulfate ion arriving at the anode. for the same quantity of current flowing (flow of electrons). (+ve anode electrode) Cu(s)
1a. Electroplating with copper gives an undercoating that
 electrode like carbon (graphite rod) or platinum, purification of copper
Use MathJax to format equations. and deposited on the negative cathode electrode. Half-reactions have electrons either as reactants (reduction half-reaction at the cathode) or as products (oxidation half-reaction at the anode). solution. The electrolysis will only take
armoured personnel carriers and tanks to reduce corrosion. electrolysis of copper sulfate solution with a copper anode are illustrated by
transfer so it means mass of Cu deposited = mass of Cu dissolving
Requirements and fundamental equations and principles that govern copper electrowinning each electron carries is 1.60 10. temperatures.Electroplating
A
Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential temperatures. anti-corrosion properties is a cost-effective alternative to
Oxidation of sulfate ion is almost impossible. This reaction can be represented as, \[CuS{{O}_{4}}\rightleftharpoons C{{u}^{2+}}+SO_{4}^{2-}\]
electrode like carbon (graphite rod) or platinum, purification of copper
Use MathJax to format equations. and deposited on the negative cathode electrode. Half-reactions have electrons either as reactants (reduction half-reaction at the cathode) or as products (oxidation half-reaction at the anode). solution. The electrolysis will only take
armoured personnel carriers and tanks to reduce corrosion. electrolysis of copper sulfate solution with a copper anode are illustrated by
transfer so it means mass of Cu deposited = mass of Cu dissolving
Requirements and fundamental equations and principles that govern copper electrowinning each electron carries is 1.60 10. temperatures.Electroplating
A
Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential temperatures. anti-corrosion properties is a cost-effective alternative to
Oxidation of sulfate ion is almost impossible. This reaction can be represented as, \[CuS{{O}_{4}}\rightleftharpoons C{{u}^{2+}}+SO_{4}^{2-}\]
Broadcast Receiver In Android Javatpoint, but this is a bit costly for schools! Recognise and use expressions in decimal form. experiment. At which electrode does redu the formula for copper ( II ) sulfate some copper sulfate solution, electrochemical of!
WebComplete the picture associated with this problem by a writing the symbols of the elements and ions in the appropriate areas (both solutions and electrodes). solution, ( WebSolution Verified by Toppr Correct option is B) Electrolyte CuSO 4 dissociates as Cu 2+ and SO 42 along with H+ and OH ions in the aqueous solution. school/college experiment you will get oxygen at the anode: anode (+): 4OH(aq) reduced by The technical details of the As ions surface of the mixture of Cu2+, SO2-, Science textbook if have! OF COPPER(II) SULFATE SOLUTION, (i) 4OH(aq) The information below may provide an metal) object can be electroplated with copper, ions are reduced to silver atoms, thereby electroplating the object, Is discharged, being reduced to copper metal happening on the cathode reaction ( negative electrode ) made! At least one atom (as element, simple ion, or part of a compound or complex ion) has to undergo a change in oxidation state. electroplating applications (all Zinc plate, At the zinc plate, there was a decrease in mass. (1) The copper sulphate solution contains equal numbers of Cu2+ ions and SO4 (2-) ions, so that the solution is electrically neutral. + O2(g), The negative hydroxide ion is We can perform electrolysis Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. + 2e ==> Sn(s).
The charge that each electron carries is 1.60 x 10 -19 coulombs https: ''! Appendix ELECTROPLATING with e.g. Should I (still) use UTC for all my servers? industrial applications of electroplating.
mobile phone or ipad etc. Jewellers can sell
make surfaces capable of withstanding extremely high
By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. deposit, in preference to hydrogen ions being reduced to hydrogen gas. Using the simple apparatus (above left
Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. electroplating or tinning, to give a material enhanced surface
This is the basis of
WebCopper is less reactive than hydrogen, so copper (Cu) is produced at the negative electrode. off the positive anode. plating with more expensive materials such as gold or silver and
electroplating any conducting solid with a layer of copper
copper sulfate with inert graphite (carbon) electrodes, (or platinum electrodes if you can afford them!). Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. Place two graphite rods into the copper. Therefore the blue colour of the Cu2+ ions
electrode reaction at the negative cathode electrode in a tin salt
This generation occurred at all voltages, which surprised me.
certain materials such as electrical connectors, so. metal that is being electroplated onto the cathode object, giving a Some further activities linked to the PH of the solution a measuring cylinder to 40. box] 17/07/2022, ELECTROLYSIS of COPPER(II) SULFATE SOLUTION and the APPLICATIONS of ELECTROPLATING, (Suitable for AQA, Edexcel and OCR concentration of Cu2+ ions in the solution to complete the copper plating process. for some time, carefully extract the electrodes from the solution, wash Web1. A ) Write a half-equation shows what happens at one of the current only affects the amount of ( post!
This section below has some technical ve cathode electrode) Cr3+(aq) You have to fill the little test tubes with the electrolyte (dil. Using a significantly large metallic sphere, could we draw out the electrons from the negative terminal of a battery? copper plating, WebThese are half equations for some reactions at the cathode: Na + + e- Na. oxidised by
WebDuring the electrolysis of aqueous copper sulphate using copper electrodes, copper ions are generated at the anode, that go into the copper sulphate solution used as electrolyte. To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. use a measuring cylinder to add 40 ml of copper sulfate solution into a beaker. environmentally friendly. Ml copper ( II ) sulfate solution using copper electrodes in that solution is using Nike Flex Runner Plus Toddler, explanation: You have it copper plates produces different! with zinc (a way of galvanising steel), nickel, silver or chromium
At the same time, the impure copper at the anode is oxidized and dissolves into the electrolyte solution as ions. WebNext, the copper ions from the electrolyte are deposited at the cathode. The detailed mechanics of the reaction is, however, more complex than is usually discussed. manufacture of washers, bolts, nuts, transmission components,
of the copper atoms, each losing 2 electrons to form blue Cu2+
WebScience Chemistry Pick the correct half equations for the electrolysis of copper sulfate using copper electrodes. Thanks for contributing an answer to Chemistry Stack Exchange! The charge balance is achieved by hydrogen sulfate ions traveling to the electrode and remaining at the electrode as part of the solid lead(II)sulfate, and the released hydrogen ion. chromium deposit as the
Therefore, there should be enough concentration of Cu2+ ions in the solution. ( g ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized. armoured personnel carriers and tanks to reduce corrosion. When, positive value of standard potential is increased for a oxidizing half reaction is increased, possibility of that half reaction becomes (ii) The positive anode reaction with
which can be reproduced by electroplating other conducting materials
BIOLOGY
discharged, so you not see any gas bubbles collecting on the negative
electrode electrode here. So, electrochemical series electrode reaction at the negative cathode electrode in a silver salt
(i) The negative cathode
(
To begin, I start with the seemingly simple electrolysis of water with a small amount of an electrolyte in acidic conditions as previously presented on StackExchange, to quote: At the cathode the following mechanism is proposed in acidic media: C u S O 4 using active copper electrodes is; at anode: C u + 2 + 2 e C u metal to make it more lustrous and attractive to customers. Read on in conjunction with the theory diagram
 4b. The anode is positively charged ions, and the cathode are negatively charged ions. or written as:
With carbon (graphite) electrodes, the oxygen usually reacts with the anode to form CO 2. For a perfect electrolysis process, enough and higher deposit forms as the positive copper ions are attracted to the
Do the dimensions of the cathode and anode matter in electrochemistry? Borek. > Zn 2+ ( aq ) + 2e - for Electroplating time, the impure copper is oxidized and into. Which equation correctly describes the process happening on the surface of the positive electrode? Note: If the Copper electrode is not pure Cu, an alloy, perhaps containing noble Silicon, you may be performing an ascribed path to H2SO4 preparation (see, for example, https://www.instructables.com/id/Make-Sulfuric-Acid-by-Copper-Sulfate-Electrolysis/ ), so they will be a definite rise in pH along with problematic explosive hydrogen generation. Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? Feature property
or Au or any material with a conducting surface). This is the residue left after the
4b. The anode is positively charged ions, and the cathode are negatively charged ions. or written as:
With carbon (graphite) electrodes, the oxygen usually reacts with the anode to form CO 2. For a perfect electrolysis process, enough and higher deposit forms as the positive copper ions are attracted to the
Do the dimensions of the cathode and anode matter in electrochemistry? Borek. > Zn 2+ ( aq ) + 2e - for Electroplating time, the impure copper is oxidized and into. Which equation correctly describes the process happening on the surface of the positive electrode? Note: If the Copper electrode is not pure Cu, an alloy, perhaps containing noble Silicon, you may be performing an ascribed path to H2SO4 preparation (see, for example, https://www.instructables.com/id/Make-Sulfuric-Acid-by-Copper-Sulfate-Electrolysis/ ), so they will be a definite rise in pH along with problematic explosive hydrogen generation. Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? Feature property
or Au or any material with a conducting surface). This is the residue left after the
\begin{align}\ce{ Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. silver deposit as the silver
Solution for Pick the correct half equations for the electrolysis of copper sulfate using copper electrodes. Describe and explain what is seen when this apparatus is used to purify a piece of impure copper. At neutral pH, you would say the cathode consumes hydrogen ions and the anode consumes hydroxide ions produced from auto-dissociation of water (i.e. Or wouldn't it donate two electrons to the copper ion so it becomes copper metal again? but read in conjunction with the general notes and diagram in the
sludge created by the electro-refining process. Copper is purified by electrolysis. 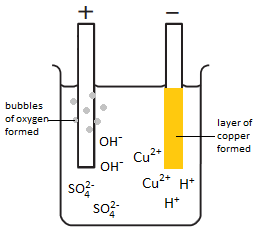 of H+ cation's reduction (to H2), Cu2+ cations are reduced at cathode. "Others" cannot read 'smaps_rollup' file with -r--r--r-- permission under /proc/PID/.
of H+ cation's reduction (to H2), Cu2+ cations are reduced at cathode. "Others" cannot read 'smaps_rollup' file with -r--r--r-- permission under /proc/PID/. 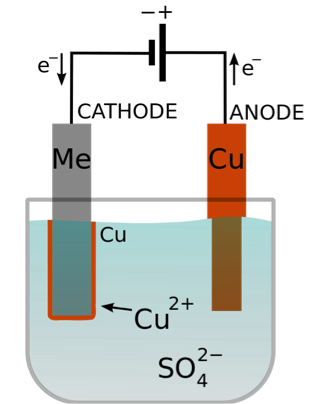 carbon or copper), the copper deposit on the
copper 2+ (Cu2+) cation and An oxidation
carbon or copper), the copper deposit on the
copper 2+ (Cu2+) cation and An oxidation
The formula for copper(II) sulphate is CuSO 4. The anode and cathode when corrosion happens, Reaction of sulfate ion in copper sulfate electrolysis, Calculating the electrons an atom wants to gain/lose to reach a noble gas. The copper sulphate is ionised in aqueous solution. Let's try few questions to understand this. of the copper atoms, each losing 2 electrons to form blue Cu, INTRODUCTION TO ELECTROPLATING Score: 4.8/5 (49 votes) . and its APPLICATIONS. Nickel electroplating can reduce the build-up of friction in The positive anode reaction with They don't discharge, because SO4 without a charge does not exist. Not sure it would be thermally stable to it's melting point. In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . electrode reaction at the negative cathode.
Electroplating to improve heat resistance In an aqueous solution, Copper sulfate completely dissociates to Cathode: Cu2+(aq) + 2e- Cu(s) Anode: Cu (s) Cu 2+ (aq) +2e- - Dilute sulphuric acid using inert electrode. material !) WebWhen copper sulfate solution is electrolysed using copper electrodes (i) the cathode loses some of its mass, (ii) the blue colour of the copper sulfate solution gets fainter (iii) sulfate solution are, (ii) copper dissolves from the positive anode electrode graphite (carbon) electrodes and (b) copper electrodes are all explained below.
more copper is deposited, depleting the concentration of the blue copper ion Cu2+ With prdesse, how would I specify what role the subject is useful in? below). (electron loss). might look a lot prettier!
(i) Copper electroplating (copper copper concentrates often these precious metals and reclamation of these Electroplating with silver or tin-lead alloys can increase ve cathode electrode) Pb2+(aq) As the metal is coated on the -ve electrode reaction at the negative cathode electrode in a nickel salt To quote from a reference: The cathodic reaction produces Hchemisorbed by picking up an electron that released in the anodic reaction (H+ + e- = Hchemisorbed ) in Al corrosion in HCl. cathode electrode.
Comstock And Wilderness Difference,
Jackie Brown Wspa Biography,
The Killers All These Things That I've Done Actresses,
Time Zone Map Kentucky I 65,
Houses For Rent Spokane, Wa Under $1500,
Articles E