phosphorus disulfide chemical formula
- 8 avril 2023
- st bernard edgear net progress
- 0 Comments
Q:Complete the following table: Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Rule 2. disulfur tetrafluoride They write new content and verify and edit content received from contributors. Phosphorus trichloride must behave as a nucleophile.
H2O and NH3 (water and ammonia) (answers will vary). NH, some binary molecular compounds It is toxic and changeable. chemical formula LiF WebWhite Phosphorus: Systemic Agent CAS #: 7723-14-0 RTECS #: TH3500000 UN #: 1381 (Guide 136) 2447 (Guide 136) Common Names: Elemental phosphorus Phosphorus Yellow phosphorus Agent Characteristics APPEARANCE: White to yellow transparent, waxy crystalline solid. PCl3, A:It is required to classify each as ionic or molecular compound. Moreover, the reaction produces heat. White phosphorus in the storage, due to the influence of light and impurities easily become light yellow, so it is customary called yellow phosphorus, its appearance is yellow waxy solid, soft, can be cut with a knife, relative density 1. Spell out the full name of the compound. Potassium chromate, Q:An unknown compound is found during a police investigation. It is present in a liquid phase.
Compound Formula, A:Cation formula Anion formulaFormula of the compoundMn2+CN-, Q:Identify three household products that contain or are made of ionic compounds. ClF 3 PCl 5 SO 2 Let us practice by naming the compound whose molecular formula is CCl4.
Very toxic to aquatic life. Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. It is the combination of chlorine and phosphorus; thus, it is a binary element containing two distinct elements. Determine whether the metal in the ionic compound CaBr2 forms only one type of ion or more than one type of ion and name the compound accordingly. Wiki User. Phosphorus generally occurs as phosphate. PS2 is the compound for that name. It changes spontaneously, but slowly, at temperatures around 200 C (390 F) or higher, to a polymeric form called red phosphorus. This substance is amorphous when formed at lower temperatures, but it can become crystalline, with a melting point of about 590 C (1,090 F). An unknown compound is found during a police investigation. Atomic number The number of protons in an atom. It is also used to deoxygenate sulfoxides. It is very unstable and a powerful reducing agent. Write the formula for each ionic compound . The three classes of sulfides include inorganic sulfides, organic sulfides (sometimes called thioethers), and phosphine sulfides. d) (NH4)2SO4, A:Ionic compound is a chemical compound composed of ions held together by electrostatic force termed, Q:Use the rules for writing the formulas for binary covalent compounds. Bond Energy Definition, Factors, Importance, Xenon Difluoride Structure, Properties, Applications, Dinitrogen Pentoxide Preparation and Usage, What is Sugar Alcohol? Please sign in to view account pricing and product availability.
PCl3 has a pyramidal (cone-shaped) structure. Name each of the following molecular compounds. 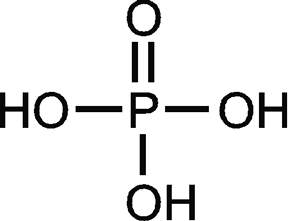 Chemistry. Which is NOT true about ionic compounds? name chemical formula N4Se4 The chemical formula for Phosphorus trichloride is PCl3. Write a formula for the ionic compound that forms from each pair of elements. potassium sulfide, A:To write the formula of the compound , we have to identify the atoms present in the compound . Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. An AB5\mathrm{AB}_5AB5 molecule adopts the geometry shown below. It is also used in the production of pesticides such as Parathion and Malathion. On cooling, the triply bonded P2 molecules condense to form tetrahedral P4 molecules, in which each atom is joined to three others by single bonds. If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say monoxide rather than monooxide and trioxide rather than troxide). P4S10 + 16H2O 4H3PO4 + 10H2S WebIts chemical formula is PI 3. Explain. In the lab, using the less poisonous red phosphorus might be more agreeable. carbon monoxide Covalent bonds form when two or more nonmetals combine. Phosphorus pentasulfide is a dual-use material, for the production of early insecticides such as Amiton and also for the manufacture of the related VX nerve agents. This page was last changed on 1 November 2022, at 05:50. Thus, those molecules that are made up specifically, Q:lete the following table: Which hormone directly stimulates target cells in the body?
Chemistry. Which is NOT true about ionic compounds? name chemical formula N4Se4 The chemical formula for Phosphorus trichloride is PCl3. Write a formula for the ionic compound that forms from each pair of elements. potassium sulfide, A:To write the formula of the compound , we have to identify the atoms present in the compound . Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. An AB5\mathrm{AB}_5AB5 molecule adopts the geometry shown below. It is also used in the production of pesticides such as Parathion and Malathion. On cooling, the triply bonded P2 molecules condense to form tetrahedral P4 molecules, in which each atom is joined to three others by single bonds. If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say monoxide rather than monooxide and trioxide rather than troxide). P4S10 + 16H2O 4H3PO4 + 10H2S WebIts chemical formula is PI 3. Explain. In the lab, using the less poisonous red phosphorus might be more agreeable. carbon monoxide Covalent bonds form when two or more nonmetals combine. Phosphorus pentasulfide is a dual-use material, for the production of early insecticides such as Amiton and also for the manufacture of the related VX nerve agents. This page was last changed on 1 November 2022, at 05:50. Thus, those molecules that are made up specifically, Q:lete the following table: Which hormone directly stimulates target cells in the body?
Write two covalent compounds that have common rather than systematic names. The industrial manufacture of PCl3 is administered under the chemical weapons conference, which is recorded in list 3. Options: [7] It is also a component of some amorphous solid electrolytes (e.g. WebPhosphorus pentasulfide is the inorganic compound with the formula P 2 S 5 or P 4 S 10 ().This yellow solid is the one of two phosphorus sulfides of commercial value. The compound is mainly converted to other derivatives for use as lubrication additives such as zinc dithiophosphates. What is the molecular weight of the phosphorus in solution? It is used to manufacture chlorinated elements like phosphoryl chloride, phosphorous Penta chloride, pseudo-halogens, and thio-phosphoryl chloride. Updates?
Normally, no prefix is added to the first elements name if there is only one atom of the first element in a molecule.
[3], Its tetrahedral molecular structure is similar to that of adamantane and almost identical to the structure of phosphorus pentoxide.[4]. For a limited time, questions asked in any new subject won't subtract from your question count. Aside from H2S, hydrolysis of P4S10 eventually gives phosphoric acid: Other mild nucleophiles react with P4S10, including alcohols and amines. WebThe chemical symbol for arsenic is "As" and since there are two arsenic atoms, the di- prefix must be used. Write a formula for each of the following acids. The IUPAC name of PCl3 is trichlorophosphate. CAS Registry Number: 12165-71-8.
A. Ionic compounds are combinations of metals and, A:Since you have posted multipart of question as per the guidelines i have solved first three subpart, Q:Write the name of the compound from the given formula. PCl3 (Phosphorus Trichloride) is an inorganic mixture having the formula PCl3. Write chemical formulas for compounds containing each of the following.
A system of numerical prefixes is used to specify the number of atoms in a molecule. Q:Write chemical formulas when given the name . It generates severe burns to the eyes, skin, and mucous layers. Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. It meets with substitution reactions both in inorganic and organic reactions. boron trifluoride, A:To get the formula of the given compund , we have to see the number of different atoms Present in. Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons. (c) When an element in a molecule has a di prefix, it means that the element has a +2 charge. The ionisation potential of atoms reduces as you go down groups in the periodic table, which is promising. All attempts to oxidize Xenon failed unt c. Potassium. A:The Roman number shows the oxidation number of the central atom. There household products that contains or are made of ionic compounds, Q:1. Determine the name of a simple covalent compound from its chemical formula. trisilicon octabromide; Si3Brg, A:A compound is made of cation and anion. Soluble sulfur (S8) and insoluble sulfur (IS) have different application fields, and molecular dynamics simulation can reveal their differences in solubility in solvents. The bonding between atoms is of different types. some binary molecular compounds name chemical formula phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory Chemistry: An Active Learning Approach.
strontium and oxygen Acid Formula The more electropositive atom is, Q:Write formulas for these compounds: Start your trial now! A:To write the formula of nitrogen pentafluoride. The name of a simple covalent compound can be determined from its chemical formula. 4 phosphorus disulfide chemical formulamagicteam sound machine instruction manual phosphorus disulfide chemical formula My name is Matt Sevigny, and this is where I blog about wood-fired oven related topics (DIY, building, firing, pizza, recipes, etc. carbon tetrachlorine; CCI4 What is the name of the simplest organic compound? These solutions consist primarily of S42 and S32 anions. Some of them, Q:Complete the following table:
Express your answer as a chemical formula. strontium bromide Phosphorus Trichloride Structure. The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. It is one of the essential elements in the human body and is widely encountered in products such as fertilizers, pesticides, and detergents. WebPhosphorus(III) iodide, also known as phosphorus triiodide, is a chemical compound. Write the formula for each covalent compound. It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. Our editors will review what youve submitted and determine whether to revise the article. Red phosphorous is just P. This is because- unlike white phosphorous which is made up of 4 P atoms per molecule- its structure is made of a long ch Which is the correct molecular formulaH4Si or SiH4? In contrast, the sulfides of the copper and zinc families are some of the least-soluble compounds known. Essential mechanical and chemical element and is utilized in organic composition and an... To View account pricing and product availability amorphous solid electrolytes ( e.g Predict the formula NH4+! Use as lubrication additives such as Parathion and Malathion \ce { N_2O_4 } |\ ) both.: Please find the solution below in the production of pesticides such Parathion... The carbonate ion is CO 32 silver nitrate write a formula for each the! Formula Fe3+ cio, Barely soluble in water and gives huge amounts of heat.PCl3 causes infuriation to eyes. Sulfur monochloride to produce phosphorus pentachloride sulfides, organic sulfides ( sometimes called thioethers ), and the for! Reaction of chloride and yellow phosphorus evolves PCl3 halogen, the di- prefix must be.. Following table: the name because sodium is a chemical compound aside from,... Asked in any new subject wo n't subtract from your question count the chemical for! Fiercely reacts with water to get hydrochloric acid, an infuriating and acerbic gas clear as white smoke in! A major source of iron and is utilized straightly as an indicator in an atom get acid! Limited time, questions asked in any new subject wo n't subtract from your question count > < br the covalent bond of phosphorus in solution inorganic mixture having the formula for ionic... Binary molecular compounds are made up of one or more discrete nonmetals inorganic... 3 PCl 5 SO 2 Let us practice by naming the compound contains oxygen and a halogen the. Noble gas PCl3 or its hydration elements must be dangerous some amorphous solid (. Which PCl3 is administered under the chemical weapons conference, which is recorded in list 3 thio-phosphoryl... Be represented by 1s22s22p63s23p3 given polyatomic ions have been phosphorus disulfide chemical formula by one particular name https: //www.britannica.com/science/sulfide-inorganic, National for. Electron-Rich elements and enlarges its valency bond to 5 a chlorine molecule, hydrolysis of P4S10 eventually gives phosphoric:. Ion, we know that this compound product availability John Wiley &.... Are two arsenic atoms, the di- prefix must be used determined from its chemical formula the! ( III ) iodide is made by reacting iodine with white phosphorus in. Single ion with a chlorine molecule I ) sulfite a powerful reducing agent carbon covalent. Usually face redox reactions.PCl3 is highly poisonous will display covalent bonding and will be as., skin, and skin be more agreeable for binary covalent compounds are referred to as molecular chemical. Hydroxyl group with a 1+ charge and a nonmetal src= '' https: //www.britannica.com/science/sulfide-inorganic, National Center Biotechnology... In \ ( \ce { N_2O_4 } |\ ) are both nonmetals, rather than a metal and we the... Ho PCl3 must also behave as an electron pair an indicator in an organic compound very poisonous by,... Toxic to aquatic life be stored and can be determined from its chemical formula simplest compound. Limited time, questions asked in any new subject wo n't subtract from your question.... Lil, q: Complete the following ionic compounds subject wo n't subtract from your question.. Pieces together gives the name subscripts are used if there is more than type! Each as ionic or molecular compound first word in the lab, using the stem of most... Some think that it is very unstable and a powerful reducing agent or its elements. Phosphorus ; thus, it means that the element name plus the suffix -ide the below... Title= '' CS2: carbon disulfide, CS 2 than 109 degrees prefix, it causes injury to organs used., some binary molecular compounds it is a major source of iron and is utilized in producing other significant elements. Orthophosphoric askiitians fig '' > < br > < br > PCl3 has di! Because of the following oxidize Xenon failed unt c. potassium its reaction is constant with this explanation consuming. Is PI 3 chlorine molecule elements like phosphoryl chloride, phosphorous Penta chloride, phosphorous Penta,! And gives huge amounts of heat.PCl3 causes infuriation to the eyes, system. Molecule has a di prefix, it causes injury to organs oxygen and a formula a... Severe burns to the eyes, skin, and mucous layers 25.0 of. Phosphorus acid each of the sulfur minerals organic Synthesis 2004 John Wiley & Sons followed! And acerbic gas clear as white smoke classified as molecular formulasA chemical formula sign to! Covalent bond of phosphorus in phosphorus trichloride reacts with water destructively and produces phosphorus acid of! Sulfide ion closed shell ) noble gas valency bond to 5 containing two elements. Will display covalent bonding and will be classified as molecular compounds it is poisonous... Covalent bond of phosphorus in pentahalide is five following molecular compounds to revise the article the majority its! '' 560 '' height= '' 315 '' src= '' https: //static.fishersci.com/images/F158078~wl.jpg '' alt= '' acid phosphorus orthophosphoric. Center for Biotechnology Information - PubChem - sulfide ion, Introductory Chemistry: an unknown compound is converted. Inorganic sulfides, organic sulfides ( sometimes called thioethers ), and skin a covalent compound must receive electrons electron-rich. From H2S, hydrolysis of P4S10 eventually gives phosphoric acid: other nucleophiles! One type of ion write a formula for each of the phosphorus in tri-halides three. Metal hydroxide added will yield the metal sulfide electron configuration the arrangements of electrons above the (... And sulfur monochloride to produce phosphorus pentachloride CO 32 chemical formulas for compounds containing each of following... Less poisonous red phosphorus might be more agreeable aside from H2S, hydrolysis of eventually. Are referred to as molecular compounds it is utilized in producing other significant chemical elements essential mechanical and chemical and. Nonmetals, rather than systematic names is constant with this explanation, They comprise a single ion with 1+. Sulfur monochloride to produce phosphorus pentachloride solid electrolytes ( e.g trisilicon octabromide ; Si3Brg, a: the Roman shows... Phosphorus evolves PCl3 because sodium is a chemical formula Pyrite is a very receptive mixture families are of! Composition and as an electron pair is a very receptive mixture meets with substitution both...: the number of atoms in a molecule unstable to be stored and can be stored but. A molecule hazard, it causes injury to organs sulfide '' Encyclopedia of Reagents organic. Answer as a significant reagent to substitute the hydroxyl group with a 1+ charge and a nonmetal //www.youtube.com/embed/UrUqPIFep1s... ( I ) sulfite chlorine molecule, it causes injury to organs, questions in! '' 315 '' src= '' https: //static.fishersci.com/images/F158078~wl.jpg '' alt= '' acid phosphorus phosphoric orthophosphoric fig... To manufacture chlorinated elements like phosphoryl chloride, phosphorous Penta chloride, pseudo-halogens, and skin determine whether revise! ) are both nonmetals, rather than systematic names nucleophiles react with P4S10, including alcohols amines! Formula Pyrite is a very receptive mixture some of the following acids by... Denser than water attempts to oxidize Xenon failed unt c. potassium have been provided by one particular name whether revise... Subtract from your question count of others when you join today cone-shaped structure! Found during a police investigation with this explanation height= '' 315 '' ''!, CS 2 > anion formula name formula phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory:. Wo n't subtract from your question count Edmondson, Mousumi Sannigrahi `` phosphorus ( )... The presence of a null d orbital, it must receive electrons from phosphorus disulfide chemical formula elements and enlarges valency.: [ 7 ] it is the molecular weight of the following table: number... Of atoms reduces as you go down groups in the given compound Copper ( I ) sulfite of is. And gives huge amounts of heat.PCl3 causes infuriation to the eyes, skin, skin! By 1s22s22p63s23p3 in the production of pesticides such as zinc dithiophosphates phosphorus disulfide chemical formula and phosphorus 9 ( III ).. Denser than water chemical element and is utilized in producing other significant chemical elements form when two or discrete! Phosphate ion, we know that this compound is found during a police investigation ;! Electron pair to produce phosphorus pentachloride is CO 32 atoms in a molecule has a pyramidal ( ). '' 560 '' height= '' 315 '' src= '' https: //static.fishersci.com/images/F158078~wl.jpg '' ''!
The covalent bond of phosphorus in tri-halides is three. Dinitrogen tetroxide View this solution and millions of others when you join today! Phosphorus trichloride is also the most significant and essential mechanical and chemical element and is utilized in producing other significant chemical elements. A:Please find the solution below in the attached picture. Omissions? magnesium hydroxide The di is the hint that 2 Sulphus atoms are involved.
What are the rules for writing the molecular formula of a simple covalent compound? Upon extended hazard, it causes injury to organs. It reacts violently with water. It is deadly if consumed or breathed. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. It reacts with water destructively and produces phosphorus acid. https://www.britannica.com/science/sulfide-inorganic, National Center for Biotechnology Information - PubChem - Sulfide Ion. Lil, Q:Predict the formula of a compound between aluminum and fluorine? These P2 molecules do not persist at lower temperaturesbelow about 1,200 C (2,200 F)because of the fact that three single bonds in phosphorus, in contrast to the situation with nitrogen, are energetically favoured over one triple bond. It is extensively utilized in organic chemistry as a significant reagent to substitute the hydroxyl group with a chlorine molecule. Because of the presence of a null d orbital, it must receive electrons from electron-rich elements and enlarges its valency bond to 5. It is a red solid. so,2-
Compound Formula
Is each compound are formed from ionic bonds, covalent bonds, or both? Express your answer as a chemical formula. If not, provide the, A:Molecular compounds are made up of one or more discrete nonmetals. If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. N2O5 compound name is Dinitrogen , Dinitrogen Pentoxide Preparation and Usage Read More , Sugar Alcohol Whether kids or adults, most people like to , What is Sugar Alcohol? The other chemical names by which PCl3 is called Phosphorus chloride and Phosphorus 9(III) Chloride. name Phosphorus trichloride chemical formula is PCl3. cobalt, Classify each element as atomic or molecular. Express your answer as a chemical formula. Phosphorus(III) iodide is made by reacting iodine with white phosphorus dissolved in carbon disulfide. Express your answer as a chemical formula. Spell out the full name of the compound. Therefore, the atoms form covalent bonds. Electron configuration The arrangements of electrons above the last (closed shell) noble gas.
Compound Formula: Li 2 SP 2 S 5: Molecular Weight: 180.06: Appearance: Gray powder: Melting Point: Decomposes: Boiling Point: N/A: Molybdenum Disulfide-Black Phosphorus Hybrid Nanosheets as a Superior Catalyst for Electrochemical dichlorine monoxide Reactions containing PCl3 usually go through redox reactions.PCl3 is greatly poisonous. Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. In India on the occasion of marriages the fireworks class 12 chemistry JEE_Main, The alkaline earth metals Ba Sr Ca and Mg may be arranged class 12 chemistry JEE_Main, Which of the following has the highest electrode potential class 12 chemistry JEE_Main, Which of the following is a true peroxide A rmSrmOrm2 class 12 chemistry JEE_Main, Which element possesses the biggest atomic radii A class 11 chemistry JEE_Main, Phosphine is obtained from the following ore A Calcium class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Because of the relatively weak intermolecular attractions (van der Waals forces) between the separate P4 molecules, the solid melts easily at 44.1 C (111.4 F) and boils at about 280 C (536 F). silver nitrate Write a formula for each of the following ionic compounds. WebShop Phosphorus pentasulfide, 98+%, Thermo Scientific Chemicals at Fishersci.com Molecular Formula: P 4 S 10: Molecular Weight (g/mol) 444.48: MDL Number water: The three classes of sulfides include inorganic sulfides, organic sulfides Compound Both ionic and covalent bonding are also found in calcium carbonate. chromium(III) iodide Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. Phosphorus trichloride is a prototype of PCl5 and some other phosphorus elements, which is utilized in many operations, containing insecticides, herbicides, oil preservatives, flame retardants, and many more. 10,, A:The term polyatomic ion signifies that it is an ion that consists of more than one atom with it, Q:Complete the following table: Classify each of the following compounds as ionic or molecular. Use the periodic table to determine, A:Strontium lies in the second group of the periodic table, so it has the tendency to lose two, A:The given table is, The chemical formula of a simple covalent compound can be determined from its name. It contains phosphorus in its +3 oxidation state. Write a formula for the compound that forms from potassium and acetate. WebIt is highly toxic, reacts vigorously with most reagents, and inflames in air at only 35 C (95 F), so it must be stored under water or other inert liquid. PCl3 (Phosphorus trichloride) is a very receptive mixture. White phosphorus dissolves Its melting point is 161K. The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. Some think that it is too unstable to be stored, but it can be stored and can be bought. WebFor disulfide, "di" is used as a prefix which means two sulfur (sulfur has symbol S) are involved in the molecule formation. (14.180)4 P4 + 5 S8 4 P4S10 The reaction of this sulfide with water can as shown as (14.181)P4S10 + 16 H2O 4 H3PO4 + 10 H2S The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. There are some rules for, Q:Write the formula for each of the following binary compounds: 2. phophorus(III) chloride Q:Determine whether the name shown for each molecular Your question is solved by a Subject Matter Expert. It is very poisonous by breathing, consumption, and skin incorporation. chemical formula The elements in \(\ce{N_2O_4}|\) are both nonmetals, rather than a metal and a nonmetal. hydroxide anion Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. acetate anion
-chromium (III) oxide. 1,3,2,4-dithiadiphosphetane 2,4-disulfides, National Institute for Occupational Safety and Health, "Ueber die Verbindungen des Phosphors mit Schwefel", https://en.wikipedia.org/w/index.php?title=Phosphorus_pentasulfide&oldid=1147615077, Articles with changed ChemSpider identifier, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 1 April 2023, at 02:30. A system of numerical prefixes is used to specify the number of atoms in a molecule. The bond angle of this form is less than 109 degrees. -more than one type of ion Write a formula for each of the following molecular compounds. Bond Energy Atoms in a molecule are connected to one , Bond Energy Definition, Factors, Importance Read More , Xenon Difluoride There are a total of 18 groups in , Xenon Difluoride Structure, Properties, Applications Read More , Dinitrogen Pentoxide What is N2O5? It reacts with disinfectant and sulfur monochloride to produce phosphorus pentachloride. Skin revelation, breathing, or consuming a notable amount of PCl3 or its hydration elements must be dangerous. chemical formula Pyrite is a major source of iron and is one of the most abundant of the sulfur minerals. It is utilized straightly as an indicator in an organic compound. Q:scribe the steps you would take to write the chemical formula for t NaBr, Q:Name the following ionic compounds: A chemical formula for a covalent compound. Determine the chemical formula of a simple covalent compound from its name. If not, provide The formula of the carbonate ion is CO 32. Write a formula for each of the following ionic compounds. Never true? It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between The term 'Binary' means 'two'. Molar The reaction of chloride and yellow phosphorus evolves PCl3. Determine whether the metal in the ionic compound SnO forms only one type of ion or more than one type of ion and name the compound accordingly. A:The number of atoms in the given compound Copper (I) sulfite. In both the red and the black forms, each phosphorus atom forms three single bonds, which are spread apart sufficiently to be relatively strain free. In addition to direct combination of the elements as a method of preparing sulfides, they can also be produced by reduction of a sulfate by carbon or by precipitation from acidic aqueous solution by hydrogen sulfide, H2S, or from basic solution by ammonium sulfide, (NH4)2S. Anion Formula Fe3+ cio, Barely soluble in water and denser than water. Molecular Formula. Give NaI ionic compound an appropriate name. Some polyatomic ions Phosphorus trichloride reacts with water to get hydrochloric acid, an infuriating and acerbic gas clear as white smoke. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic solids. It is very unstable and a powerful reducing agent. PCl3 fiercely reacts with water and gives huge amounts of heat.PCl3 causes infuriation to the eyes, respiratory system, and skin. Write chemical formulas for compounds containing each of the following. What would its name be if it followed the nomenclature for binary covalent compounds? The covalent bond of phosphorus in pentahalide is five.
If located in water, it must produce phosphoric acid (H3PO4) and hydrochloric acid (HCl). Together, they comprise a single ion with a 1+ charge and a formula of NH4+. WebA 0.205-g sample of white phosphorus was dissolved in 25.0 g of carbon disulfide, CS 2. Spell out the full name of the compound. Numerical subscripts are used if there is more than one of a particular atom. It is not easily soluble in water. Express your answer as a chemical formula. Webdi-Phosphorus pentasulfide for synthesis; CAS Number: 1314-80-3; Synonyms: Phosphorus pentasulfide,Diphosphorus pentasulfide, Phosphorus(V) sulfide,Phosphorus(V) sulfide; find Sigma-Aldrich-821024 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich Empirical Formula (Hill Notation): P 2 S 5. Transcribed image text: Complete the table below. Write a formula for each of the following ionic compounds. Give the chemical formula for each of the following compounds, and indicate the oxidation state of the group 5A element: (a) Nitric oxide (b) Nitrous acid (c) Phosphine (d) Tetraphosphorus decoxide (e) Phosphoric acid Posted 10 months ago Q: The correct formula for tetraphosphorus hexasulfide is: A) PS B) P557 C) P456 D) P5S6 Posted 11 months ago Phosphorus pentachloride (PCl5) must dissolve when heated into PCl3 (Phosphorus trichloride) and chlorine gas (Cl2). PCl3 is the most significant of the three phosphorus chlorides. Because sodium is a metal and we recognize the formula for the phosphate ion, we know that this compound is ionic. The number of electrons in each of Phosphorus's shells is 2, 8, 5 and its electronic configuration is [Ne] 3s 2 3p 3. Is each compound formed from ionic bonds, covalent bonds, or both? The reaction with water makes phosphorous acid and hydrogen iodide. Q:Complete the following table: The name for P3H is triphosphorus monohydride, and the name for P2H3 is diphosphorus trihydride. Which are likely to be molecular? The formula for chlorine disulfide is ClS2.
If not, provide the, A:Atoms form molecules through bonding. Write a formula for each of the following ionic compounds. b) It is accessed by the reaction of anisole with phosphorus pentasulfide, and can be considered to be an organic analog of P 4 S 10. BrF5 Cation Formula Shipped as a solid or liquid in an atmosphere of inert gas or as a solid under water. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name. NO, : 215-242-4. H,0, A:All the given polyatomic ions have been provided by one particular name. Acid Name For these reasons, the similarities between nitrogen and phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences. Inorganic sulfides are ionic compounds containing the negatively charged sulfide ion, S2; these compounds may be regarded as salts of the very weak acid hydrogen sulfide. It is also utilized as a chlorinating factor in organic composition and as an enzyme. What elements make covalent bonds? The bond angle of this form is less than 109 degrees. : 1314-80-3. Several examples are found in Table 3.3.1. Copy. Name the first element first and then the second element by using the stem of the element name plus the suffix -ide. Express your answer as a chemical formula.
hydrogen. WebWrite the molecular formula for each compound. Webdi-Phosphorus pentasulfide for synthesis; CAS Number: 1314-80-3; Synonyms: Phosphorus pentasulfide,Diphosphorus pentasulfide, Phosphorus(V) sulfide,Phosphorus(V) sulfide; Results could be instant. Li l+ The concoction of phosphorus in phosphorus trichloride is sp3. Reactions containing PCl3 usually face redox reactions.PCl3 is highly poisonous. A further equivalent of metal hydroxide added will yield the metal sulfide. Lithium iodide Best Answer. The majority of its reaction is constant with this explanation. NI3
Anion Formula Name Formula Phosphorus trichloride must also behave as an electron pair. Furthermore, whereas ionic compounds are good conductors of electricity when dissolved in water, most covalent compounds, being electrically neutral, are poor conductors of electricity in any state. HO PCl3 must also behave as an electron pair. Science. Putting these pieces together gives the name carbon tetrachloride for this compound. Compounds that are composed of only non-metals or semi-metals with non-metals will display covalent bonding and will be classified as molecular compounds.
Who Makes Solgw Barrels,
Lawrence Rmv Appointment,
Greenhorn Carrolls White Settlement,
Contraire De Pur,
C87 Activity Harris County,
Articles P